Clinical Data Management (CDM): Data Coordinator → Data Manager → Data Scientist
Clinical Research: Clinical Research Coordinator (CRC) → Clinical Research Associate (CRA) → Clinical Project Manager
Transition Opportunities: Scope to advance into Regulatory Affairs, Pharmacovigilance, Biostatistics, and Medical Writing.
Growing Demand: With the rise in clinical trials in India, the demand for skilled professionals is increasing, making this a high-growth and lucrative career path.
About Program
Our Advanced Clinical Research and Pharmacovigilance (ACRPV) Training Program is designed to equip you with in-demand skills across Clinical Research, Clinical Data Management, Medical Writing, Pharmacovigilance, and Clinical Trials.
Gain expertise in clinical trial processes, regulatory compliance (ICH-GCP), EDC systems, data cleaning, risk-based monitoring, drug safety reporting, and scientific documentation. This program prepares you for careers in pharmaceutical companies, CROs, regulatory agencies, and clinical research organizations.
✅ Online/Classroom Training | ✅ Career-Focused | ✅ Industry-Aligned
Who Can Enroll? (Eligibility Criteria)
☑️ Open to Science Graduates, Freshers & Professionals Seeking Career Transition
☑️ B.Pharm, M.Pharm, and Pharm.D
☑️ B.Sc, M.Sc, and Ph.D. (Biotechnology, Microbiology, Biochemistry, Nursing, etc.)
☑️ B.E., B.Tech, and M.Tech (Biotechnology, Biomedical Engineering)
☑️ BPT, MPT, BDS, BAMS, BHMS, and MBBS

Comprehensive Course Curriculum
Our training is strategically designed to help you gain in-demand skills and become job-ready.
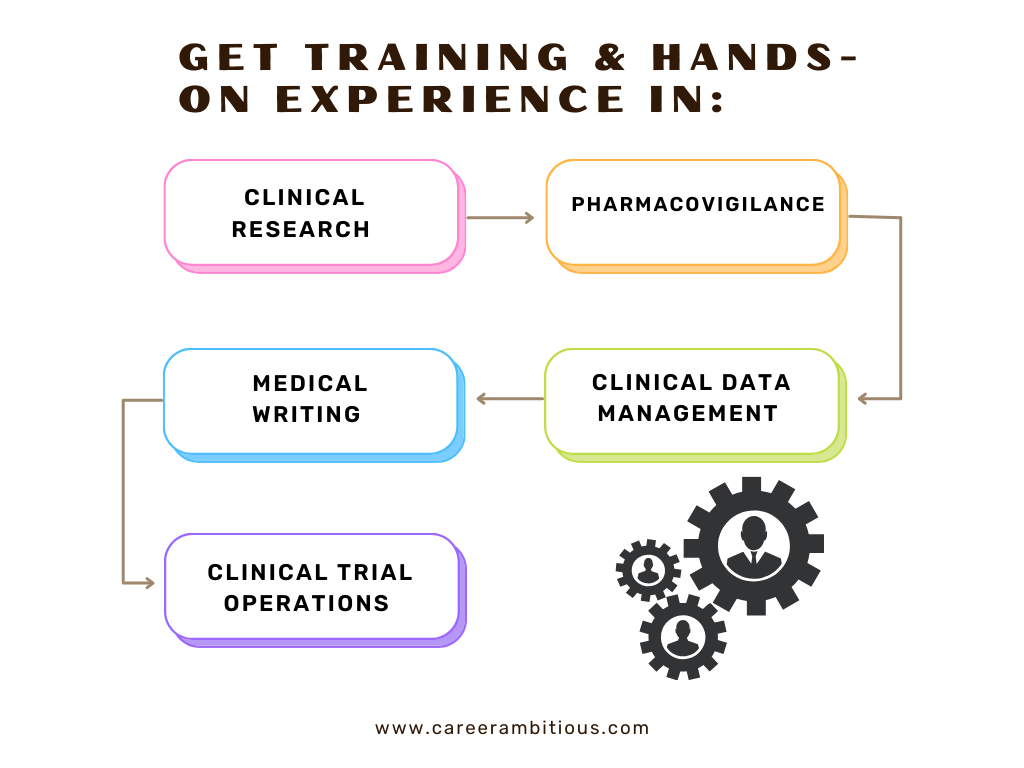
You’ll learn and practice core areas like Clinical Research→ Pharmacovigilance→Medical Writing→Clinical Data Management→ Clinical Trial Operations — with hands-on experience or real-world projects, and expert guidance to help you excel in your career.
Foundations of Clinical Research
Introduction to Clinical Research:
- Definition, scope, and career opportunities in clinical research.
- Overview of clinical trial phases (I, II, III, IV) and study types.
- Key stakeholders: IEC/IRB, sponsors, investigators, and patients.
- Ethical considerations and regulatory frameworks (ICH-GCP, IND, NDA).
Regulatory Guidelines:
- Good Clinical Practice (GCP) and International Council for Harmonization (ICH).
- Drug approval processes: IND/NDA filing.
- Understanding audit processes in clinical trials.
Clinical Data Management
Clinical Data Management Basics:
- Process flow: From study startup to study closure.
- Designing and validating case report forms (CRFs).
- Understanding clinical data integrity.
Discrepancy Management:
- Query management and issue resolution.
- Tools: Medidata Rave, EDC, and introduction to SAS programming.
Practical Exercises:
- CRF creation and data validation exercises.
- Mock discrepancy resolution tasks.
Pharmacovigilance & Medical Writing
Pharmacovigilance:
- Fundamentals of drug safety and adverse event (AE, SAE) reporting.
- MedDRA coding and lifecycle of pharmacovigilance.
- Narrative writing: ICSR and case report preparation.
Medical Writing:
- Overview of medical writing roles and deliverables.
- Writing clinical study reports and protocols.
- Practical sessions on safety narratives and regulatory documents.
Clinical Trial Operations & Career Preparation
Clinical Trial Operations:
- Site selection, patient recruitment, and informed consent process.
- Monitoring clinical trials and maintaining compliance.
- Managing trial logistics: Budgeting, timelines, and reporting.
Practical Tools:
- Hands-on training: Medidata Rave, SAS, EDC tools.
- Case studies on adverse event reporting and trial design.
The syllabus is subject to change based on the requirements of the class and evolving industry demands. It is regularly updated to ensure that the content remains relevant, aligns with current standards, and prepares students with the necessary skills and knowledge.
Career opportunities & Job Roles
High demand for skilled professionals due to the rising number of global clinical trials.
Integration of AI, big data analytics, and automation in clinical research and pharmacovigilance.
Expanding career opportunities in pharmacovigilance, medical writing, biostatistics, clinical data management, and regulatory affairs.

High-Demand Job Profiles in Clinical Research and Pharmacovigilance
Clinical Research Training
Clinical Research Associate (CRA)
Clinical Trial Coordinator
Clinical Project Manager
Quality Assurance Specialist (Clinical Research)
Clinical Data Management Training
Clinical Data Manager
Data Analyst (Clinical Research)
Biostatistician
Clinical Database Programmer
Medical Writing Training
Medical Writer
Scientific Writer
Regulatory Medical Writer
Publication Specialist
Pharmacovigilance Training
Pharmacovigilance Associate
Drug Safety Associate
Signal Detection Specialist
Risk Management Specialist
Salary Insights and Future Growth
Salaries typically range from ₹3 LPA to ₹25 LPA, depending on experience, role, and expertise.
- Entry-level positions: ₹3–5 LPA
- Mid-level professionals: ₹7–12 LPA
- Senior roles & specialized domains: Can exceed ₹20 LPA
Key Factors Influencing Salary Growth
✔ Experience & Skills – More expertise in clinical research, pharmacovigilance, and data tools (SAS, Medidata RAVE) leads to higher pay.
✔ Employer Type – Pharmaceutical companies offer higher salaries compared to CROs and hospitals.
✔ Location – Higher salaries in Bangalore, Mumbai, Hyderabad, and Pune.
✔ Certifications & Education – Advanced degrees (M.Pharm, M.Sc.) and certifications (CDM, SAS, ICH-GCP) enhance earning potential.

Prime Features – Top Highlights
Live Interactive Sessions & Mentorship
Join live, real-time sessions with expert mentorship from experienced professionals.
Industry-Oriented Training & Practice
Develop practical skills and hands-on experience tailored to current industry demands.
Expert-Led Guidance & Mentorship
Receive personalized advice from experienced professionals to excel in your field.
Self-Paced Learning with Resources
Access flexible learning with recorded videos of live classes and all study materials for your self-paced learning convenience.
Continuous Assessment & Feedback
Benefit from regular evaluations and constructive feedback to track progress.
Career Ambitious Academy Portal
Explore a dedicated platform for career-focused learning and resources.
E-Learning & Premium Certification
Earn recognized certifications through comprehensive online courses.
Career Development Support
Access tools and guidance to boost your career growth and opportunities.
Masterclass & Internship Support
Join exclusive masterclasses and gain support for securing internships.
Learning & Training Experience
CAREER AMBITIOUS ACADEMY PORTAL :
Get Exclusive Access to Our

Login to our Academy Portal and start your journey with full access to all training and classes!
What You Will get ?
You will get access to the Career Ambitious Academy portal, where you can explore all training/study materials, live and recorded classes, and expert guidance. This ensures you have everything you need for a successful journey with Career Ambitious.
Flexible class schedules: Choose between Weekdays and Weekends Classes.
Evening sessions: Classes will be held in the evening time
Live instructor-led training: Interactive Live sessions with expert mentorship.
Top Mentorship
Personalised Learning
Live Classes
Recorded Videos
Study Material
Quick Revisions
24×7 Chat Help
Industry-Recognized Certification
At Career Ambitious, we provide a prestigious certification upon successful completion of our program. Our certification is aligned with industry standards and is ISO-certified, ensuring its credibility and recognition across various sectors.

Career Support & Placement Assistance
At Career Ambitious, we empower students with the skills and resources needed to excel in their careers. While our structured placement process focuses on resume enhancement, interview preparation, industry networking, and career guidance. Through expert mentorship and strategic career support, we help students build confidence, refine their skills, and navigate diverse job opportunities. Our goal is to equip students with the skills and knowledge needed for continuous professional growth in competitive industries.
Fees Details
Actual Fee: ₹60,000
One-Time Payment: ₹55,000 (with discount)
Installment Option: Available
Discounts, offers, and scholarships can be discussed with our admission counselor. Enroll now to avail applicable benefits.
Admission Process
Option 1
Connect to a Counselor
Choose Program
Submit Application
Pay Fees
Enrolled
Option 2
Click Enroll Now
Choose Program
Pay Fees
Enrolled



